
EUnetHTA JA3
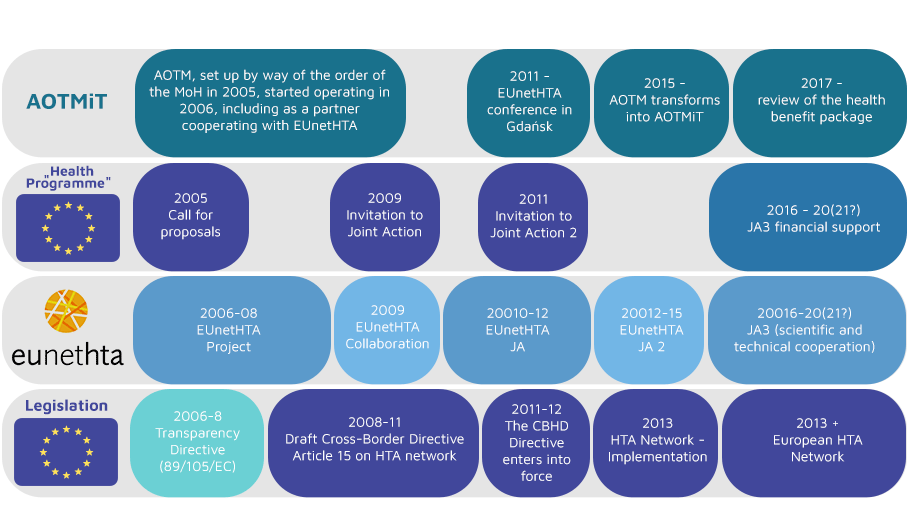
The Agency for Health Technology Assessment and Tariff System has been involved in the European network for Health Technology Assessment (EUnetHTA) since its inception in 2005.
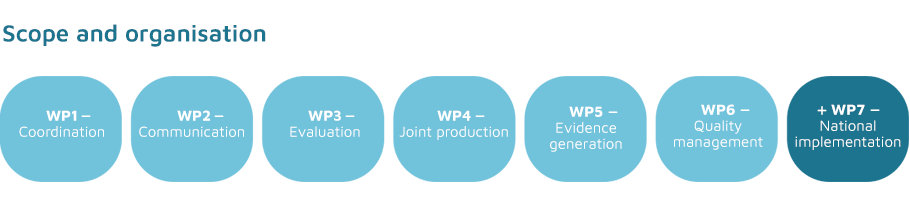
Scope of the Project
Austria
- GOG – Gesundheit Österreich GmbH/Geschäftsbereich
- HVB – Hauptverband der Österreichischen Sozialversicherungsträger (Association of Austrian Social Insurance Institutions)
- AIHTA – Austrian Institute for Health Technology Assessment
- UMIT – University for Health Sciences, Medical Informatics and Technology
Belgium
- IPH – Scientific Institute of Public Health
- KCE – Belgian Health Care Knowledge Centre
- RIZIV-INAMI – Rijksinstituut voor Ziekte- en Invaliditeitsverzekering
Bulgaria
- NCPHA – National Center of Public Health and Analyses
Croatia
- MIZ – Ministry of Health of the Republic of Croatia
- CHIF – Croatian Health Insurance Fund
- CIPH – Croatian Institute of Public Health
Cyprus
- MoH Cyprus – Ministry of Health of Cyprus
Czech Republic
Denmark
- DEFACTUM (formerly CFK) – DEFACTUM
Estonia
- UTA – Institute of Family Medicine and Public Health
Finland
- FIMEA – Finnish Medicines Agency
- FinCCHTA – Finnish Coordinating Center for Health Technology Assessment
- THL – National Institute for Health and Welfare
France
- HAS – French National Authority for Health (Haute Autorité de Santé)
Germany
- DIMDI – German Institute for Medical Documentation and Information
- GBA – Gemeinsamer Bundesausschuss
- IQWIG – Institute for Quality and Efficiency in Health Care
Greece
- EKAPTY-NKUA – National and Kapodistrian University of Athens
- EKAPTY SA – National Evalution Center of Quality and Technology in S.A.- EKAPTY
- EOF – National Organization for Medicines
- EOPYY – National Organisation for Healthcare Provision
- IFET – Institute of Pharmaceutical Research and Technology
- OCSC – Onassis Cardiac Surgery Centre
Hungary
Ireland
- HIQA – Health Information and Quality Authority
- NCPE – National Centre for Pharmacoeconomics, St. James Hospital
Italy
- Agenas – National Agency for Regional Health Services
- AIFA – Italian Medicines Agency
- CRUF/AOUIVR – Centro Regionale Unico sul Farmaca del Veneta
- DGFDM IT – Sede del Ministro – Ministero della salute
- RER – Regione Emilia-Romagna
- UCSC GEMELLI – University Hospital A. Gemelli
- UVTA/AOP – Unita di Valutazione Technology Assessment
- Veneto/CRUF – Regione Del Veneto – Area Sanita E’ Sociale
Latvia
- NVD – National Health Service
Lithuania
Malta
- DPA/MoH Malta – Directorate for Pharmaceutical Affairs
Netherlands
Norway
- Hdir – Norwegian Directorate of Health
- NIPHNO (formerly NOKC) – The Norwegian Institute of Public Health
- NOMA – Norwegian Medicines Agency
Poland
- AOTMiT – Agency for Health Technology Assessment and Tariff System
Portugal
- ACSS IP – Administração Central do Sistema de Saúde, I.P.
- INFARMED – National Authority of Medicines and Health Products
Romania
- NIPHB – Institutu National De Sanatate Publica (INSP)
- NSPHMPDB – National School of Public Health, Management and Professional Development
- UBB – Babes-bolayi University, Cluj School of Public Health
Slovakia
- MoH Slovak Republic – Ministry of Health of the Slovak Republic
- UniBA FOF – Comenius University in Bratislava
Slovenia
- JAZMP – Public Agency of the Republic of Slovenia for Medicinal Products and Medical Devices
- MoH Slovenia – Ministry of Health of the Republic of Slovenia
- NIJZ – National instute of Public Health (NIJZ)
Spain
- AEMPS – Agencia Española de Medicamentos y Productos Sanitarios
- AETS-ISCIII – The Instituto De Salud Carlos III
- AETSA – Andalusian HTA Agency
- AQuAS – Agency for Health Quality and Assessment of Catalonia
- AVALIA FNS – Fundacion Profesor Novoa Santos
- AVALIA-T – Galician Agency for HTA
- BIOEF – Basque Foundation for Health Innovation and Research
- DGFPS MSPSI – Directorate General for Pharmacy and Health Care Products
- FPS – Fundación Pública Andaluza Progreso y Salud
- Funcanis – Fundación Canaria de Investigación Sanitaria
- Osteba – Basque Office for Health Technology Assessment- Ministry for Health
- SESCS – Evaluation AND Planning Unit – Directorate of the Canary Islands Health Service
Sweden
- MPA – Medical Products Agency
- SBU – Swedish Agency for Health Technology Assessment and Assessment of Social Services
- TLV – Dental and Pharmaceutical Benefits Agency
Switzerland
- SNHTA – Swiss Network for HTA
Ukraine
- MoH Ukraine – HTA Department of SEC of Ministry of Health of Ukraine
Great Britain
- AWTTC – All Wales Therapeutics and Toxicology Centre
- HIS – Healthcare Improvement Scotland
- NICE – National Institute for Health and Care Excellence
Objective of the Project
Define and implement a sustainable and balanced model of cooperation on HTA at European level.
The End Date – May 2021 (extended duration).
Description of the Project
- creating joint clinical assessments;
- providing scientific consultations to drug manufacturers;
- collecting post-registration data;
- developing methodological guidelines and procedures.
The Agency is actively participating in the organisation, and in 2019-2020, as part of the project it has developed:
- as a Co-author: Report on ustekinumab in ulcerative colitis UC;
- as a Reviewer: Report on brolucizumab in AMD and Report on sotagliflozin in diabetes;
- it also reviewed the methodological guidelines: Critical assessment of economic evaluations;
- progress in the work concerned, in addition: the work in working groups: Future Model of Cooperation on HTA and Common Phrases, co-creation of SOPs (SOP on Data Extraction) and participation in Information Specialists Pool.
Materials to download:
Report on brolucizumab in AMD (Brolucizumab for the treatment of adults with neovascular (wet) age-related macular degeneration (AMD))
Report on sotagliflozin in diabetes (Sotagliflozin for adult patients with Type 1 Diabetes Mellitus who have inadequate blood glucose control using insulin or insulin analogues)
The benefits of cooperation:
- opportunity to work together and exchange experiences with almost all European HTA agencies;
- ongoing monitoring of the HTA development trends;
- obtaining access to data provided to EUnetHTA by the cooperating MAHs;
- networking.
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2021]

