The aim of the project is to develop HTA guidelines for medical devices, taking into account the specifics of the different groups of intended use and safety classes of devices. While guidelines for the HTA for medicines are well established worldwide, the diversity and short lifespan of medical devices means that few guidelines have so far been developed for these technologies (in Europe, guidelines dedicated to the assessment of medical devices have been developed only in France, Sweden and the UK).
As part of the project, a document titled – Review of the current state of knowledge and solutions (Przegląd aktualnego stanu wiedzy i rozwiązań), was prepared and the Agency’s work is based on editing and adapting the current “Health technology assessment guidelines (version 3.0)”. Due to the diversity of medical devices, the key assumption for the project is that Analiza Problemu Decyzyjnego it is to provide the groundwork for the preparation of further elements of the HTA report and to justify the decision to consider the device in the given process.
The first guidelines under development, applicable to the current reimbursement procedure (which requires the manufacturer to submit relevant analyses for new products) will focus on the assessment of medical devices which are:
- therapeutic;
- manufactured in series;
- used individually by the patient (not requiring assistance or participation of healthcare professionals);
- available in pharmacies.
Other groups of medical devices (i.e. diagnostic devices, implantable devices) will be successively covered by the guidelines at further stages of the project – due to their use and safety profile, it may be necessary to modify the requirements or conduct additional analyses.
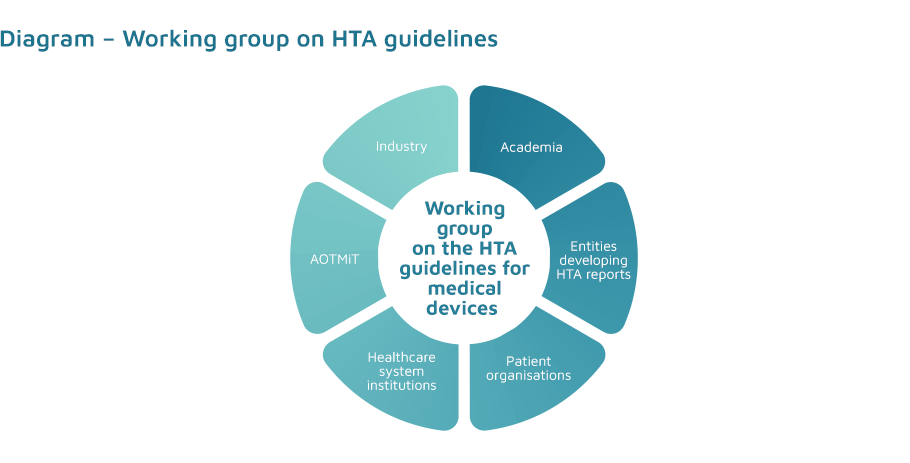
The team involved in the development of the guidelines is made up of 81 people, including:
- industry representatives;
- HTA report developers;
- academia;
- patient organisations.
The inaugural meeting took place in July 2019 and, after collecting initial proposals for amendments to the document titled “Health technology assessment guidelines” and establishing the wording of the guidelines for decision problem analysis, the works on the project have gained momentum in Q2 2020, when the team met regularly once or twice a week.
Correspondence:
In connection with the process of finalizing the work on the HTA Guidelines for medical devices, the Agency publishes responses to the comments provided in official correspondence:
- Stanowisko Izby Gospodarczej „Farmacja Polska” z dnia 7 kwietnia 2021 r.
- Odpowiedź Agencji z dnia 5 maja 2021 r. na stanowisko Izby „Farmacja Polska”
- Stanowisko Ogólnopolskiej Izby Gospodarczej Wyrobów Medycznych POLMED z dnia 14 kwietnia 2021 r.
- Odpowiedź Agencji z dnia 5 maja 2021 r. na stanowisko Izby POLMED
- Stanowisko Organizacji Pracodawców Przemysłu Medycznego TECHNOMED z dnia 14 kwietnia 2021 r.
- Odpowiedź Agencji z dnia 5 maja 2021 r. na stanowisko Organizacji TECHNOMED
Documents to download:
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2021]

