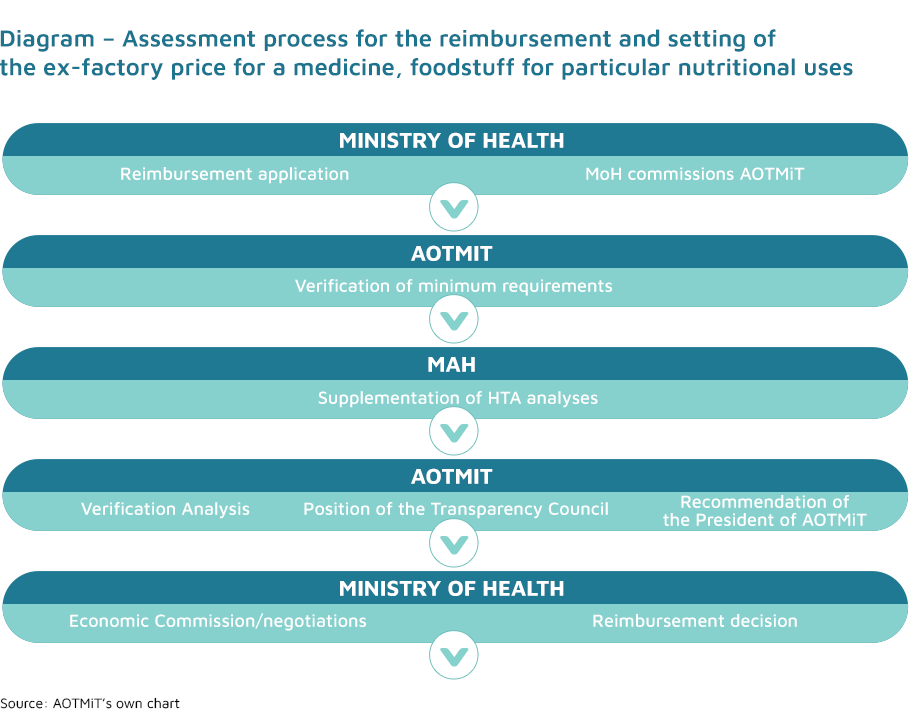
The inclusion of medicines, foodstuffs for particular nutritional uses and medical devices on the list of reimbursed medicines to be used within the scope of the indications included in their marketing authorisation, or the setting/modification of the ex-factory price of a medicine is based on a relevant application submitted to the Ministry of Health by a representative of the marketing authorisation holder for the health technology in question.
The recommendation of the President of the Agency is one of the criteria considered by the Minister of Health when issuing an administrative decision on the reimbursement and setting of the ex-factory price. Recommendations are based on the Agency’s verification analysis of the HTA documentation attached to the application, as well as the position of the Transparency Council. The method and procedures of preparation of AOTMiT’s verification analysis and the fee for this analysis are specified in the regulation of the Minister of Health of 18 December 2013 (Journal of Laws 2014, item 4).
The Agency’s assessment includes:
- verification whether the analyses attached to the application comply with provisions of the regulation of the Minister of Health of 2 April 2012 on minimum requirements to be met by the analyses included in the applications for reimbursement and setting the ex-factory price and for increasing the ex-factory price of a medicine, foodstuff for particular nutritional uses and medical device which have no reimbursed equivalent in an indication in question (Journal of Laws of 2012, item 388);
- assessment of the clinical analysis, economic analysis, budget impact analysis and, in specific cases, the rationalisation analysis carried out by:
– analysing scientific literature, in particular clinical practice guidelines and publications describing the results of scientific research – including clinical trials, systematic reviews and economic analyses,
– requesting the opinion of experts,
– analysing documents setting out the conditions of marketing authorisation as well as the method and level of financing of medicines, foodstuffs for particular nutritional uses and medical devices,
– making queries in bibliographic databases – to the extent relevant for the subject of the analyses assessment,
– verifying the calculations constituting the basis for the estimates presented in the analyses in question, and if they are deemed incorrect, making own calculations in this respect,
– identifying discrepancies between the information included in the analyses in question and the actual status as at the date of application submission;
- carries out a review of reimbursement recommendations concerning the medicine, foodstuff for particular nutritional uses and medical device in question from other countries, together with an analysis of their justifications and detailed conditions for reimbursement;
- carries out an analysis of the detailed conditions for the reimbursement of a medicine, foodstuff for particular nutritional uses and medical device in other countries, which is carried out by comparing information on: the current level of reimbursement in other countries;
- determines the threshold value of the ex-factory price of a medicine, foodstuff for particular nutritional uses and medical device, at which the cost of obtaining an additional quality-adjusted life year (QALY), and in case it is not possible to determine this cost – the cost of obtaining an additional life year, equals the threshold value, determined as three times the gross domestic product per one inhabitant.
The Agency’s verification analysis is promptly forwarded by the President of the Agency to the Transparency Council and the applicant, and is then published in the Public Information Bulletin along with the applicant’s analyses. Remarks may be submitted to all the above-mentioned analyses within 7 days from the date of their publication.
On the basis of the position of the Transparency Council on the assessment of a medicine, foodstuff for particular nutritional uses or medical device, the President of the Agency issues a recommendation as to whether it is justified or not to reimburse the particular medicine, foodstuff for particular nutritional uses and medical device.
The recommendation is sent by the President of the Agency to the Minister of Health within not more than 60 days from the date of receiving the application for reimbursement and setting the ex-factory price or increasing the ex-factory price of the medicine, foodstuff for particular nutritional uses and medical device. If the application does not meet the requirements specified in the regulation of the Minister of Health on minimum requirements, the President of the Agency requests the applicant to supplement the analyses in 21 days to complete the documentation. In such cases, all of the Agency’s deadlines are suspended.
The Agency’s verification analysis is forwarded to the Economic Commission by the Minister of Health along with the above-mentioned application, the position of the Transparency Council, the recommendation of the President of the Agency and other documents on the basis of which the recommendation was prepared, in order to conduct negotiations on the reimbursement conditions.
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2021]

