Import for individual needs involves importing medicines and foodstuffs for particular nutritional uses which do not have a marketing authorisation in Poland but are necessary to save a patient’s life or health.
The aforementioned medicines may be imported under the following conditions:
- the medicine is approved for sale and has a market authorisation in the country from which it is imported,
- the medicine does not have an equivalent in Poland – a medicine that contains the same active substance, pharmaceutical form and dosing,
- the medicine has not been the subject of proceedings in which the President of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products refused its marketing authorisation, renewal of such authorisation or in which the marketing authorisation was withdrawn.
Import for individual needs is possible provided that one has:
- a request form issued at a hospital, or
- a request form issued by a physician providing treatment in outpatient settings, confirmed by a consultant in a relevant branch of medicine.
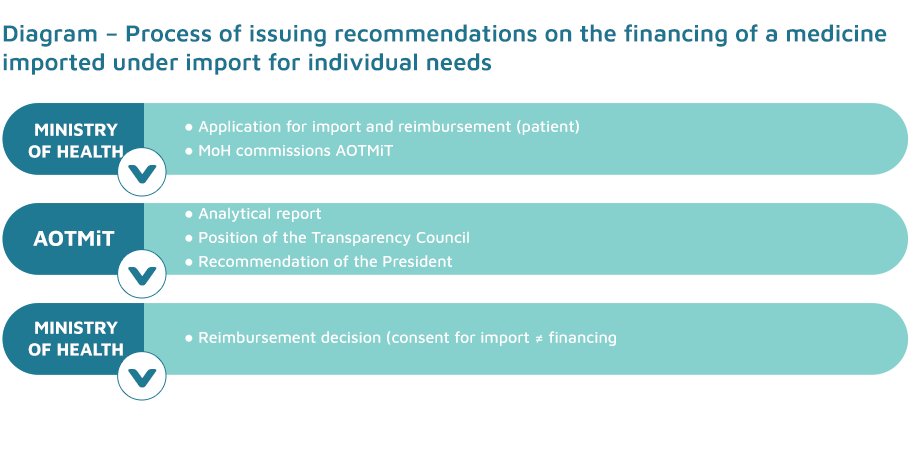
A medicine without a marketing authorisation or not available for sale in Poland and imported under the import for individual needs procedure as well as foodstuffs for particular nutritional uses are made available to the patient for a flat-rate fee for the unit packaging if the Minister of Health consents to the reimbursement.
The reimbursement application for a medicine or foodstuff for particular nutritional uses is considered by the Minister of Health within 30 days from the date of application.
When considering the application, the Minister of Health takes into account the following criteria:
- the severity of the clinical condition covered by the reimbursement application;
- the product’s efficacy and effectiveness;
- the product’s safety of use;
- the relationship between health benefits and risks of use;
- the product’s price competitiveness;
- the impact on budgets of both the National Health Fund and the patient;
- the existence of an alternative health technology, within the meaning of the Act on healthcare services, its efficacy, safety of use, recommendation of the President of the Agency,
(if one has been issued) and other opinions, in particular those of national or regional consultants, obtained during the process of application consideration.
To assess the validity of issuing a consent for the reimbursement of a given medicine or foodstuff for particular nutritional uses, the Minister of Health may commission the President of the Agency for Health Technology Assessment and Tariff System to issue a recommendation on the justifiability of the reimbursement of the medicine or foodstuff for particular nutritional uses in question in a particular indication. It is obligatory to obtain a recommendation of the President of the Agency in the event that the Ministry receives more than 10 applications for granting consent to the reimbursement of a medicine containing a specific active substance or a foodstuff for particular nutritional uses of a specific composition in a particular indication.
In the event that the Minister commissions the President of the Agency to issue a recommendation on the reimbursement of a medicine or a foodstuff for particular nutritional uses under the import for individual needs procedure, the time limit for considering the case is suspended until the Minister receives the President’s recommendation. Such a recommendation is valid for 3 years and is also applicable to other medicines containing the same active substance and a similar dosage form, as well as to foodstuffs for particular nutritional uses with a composition identical to that of the product in question.
After receiving a commission from the Minister of Health, the President of the Agency performs an assessment of the healthcare service and prepares a full or abridged report, which is immediately forwarded to the Transparency Council, which then prepares a position for the President of the Agency.
In view of the reimbursement criteria taken into consideration by the MoH, for products imported under the import for individual needs procedure, the report contains, i.a., the following:
- a description of the healthcare service covered by the commission, with particular reference to the availability of alternative healthcare services dedicated for the disease, health condition or indication in question;
- a description of the disease, health condition or indications in which the healthcare service covered by the commission is provided;
- an indication of scientific evidence in the form of secondary studies or clinical practice guidelines;
- data on the costs of the healthcare service and its components.
A negative recommendation of the President of AOTMiT regarding the validity of authorising the reimbursement from public funds of a given product imported as part of the import for individual needs procedure in the indication in question, or the President of the Agency’s recommendation on the justifiability of reimbursement with regard to the active substance contained in the medicine in question or with regard to the foodstuff for particular nutritional uses in question, constitute, i.a. the basis for the Minister of Health to refuse reimbursement of products imported under the import for individual needs procedure in relation to the medicine or foodstuff for particular nutritional uses in a particular indication.
*Ladies and Gentlemen
We inform you that the materials and results of the discussions posted on the Agency’s website are the result of the conceptual work and analytical process carried out by the team of the Agency for Health Technology Assessment and Tariff System based on the EBM paradigm, including: search, selection, synthesis and interpretation of scientific evidence, or the data analysis carried out.
In connection with the above, we would like to inform you that the use of analytical material or the results of the discussion, in accordance with good practice, should be accompanied by information on the source in the form: [title of presentation / report], AOTMiT, Warsaw, June 2021]

